In this blog, you’ll get in-depth insights into how image-based flow cytometers work and why they’re highly popular in modern applications.
Drawbacks of conventional flow cytometers
Conventional flow cytometer has the advantage of high throughput: detect single cell properties at rates from hundreds to 100,000 cells per second. This makes conventional cytometers good for studying high-volume cell populations. Also, while a conventional flow cytometer helps in multi-parametric analysis, it lacks spatial resolution.
What does this mean?
These devices find it tough to distinguish particles or cells close in size as the spatial resolution is determined by the size of the laser beam and the optics used to detect scattered light. Hence, the ability of traditional flow cytometers to discriminate between particles of similar size becomes challenging. It can lead to overlapping signals and difficulty in accurately identifying distinct cell populations – resulting in inaccurate data analysis. Consequently, this hampers the reliability of results and hinders proper understanding of complex biological systems.
Rise of image-based flow cytometers
Images convey valuable information about cell shape, size, morphology, and distribution of labeled biomolecules within cells. Incorporating imaging capabilities into conventional flow cytometry is highly recommended since cellular morphology analysis is crucial for many biological studies and clinical diagnoses, including cancer screening. In addition, further detailed imaging of each cell can better differentiate between cells and debris to give quantitative visualisation of cells with great spatiotemporal resolution.
In clinical diagnostics and cell biology, the imaging strategies (such as the speed of the laser scanning technologies used in laser confocal microscopy), the frame rate of the image sensors, the complexity of the image processing, the computing power and optical blur brought on by cells moving at fast linear speeds are what primarily limit the imaging throughput. These restrictions make it difficult to do analysis and large-scale single-cell imaging.
What is Image Flow Cytometry (IFC)?
Image Flow Cytometry (IFC) has been made possible by recent advancements in image technology, electronics, and digital computers. IFC combines the single-cell identification and high throughput of traditional flow cytometry with the cell image collection capabilities of fluorescence microscopy. As a result, it becomes the perfect method for carrying out both morphological characteristic analysis and phenotypic characterisation of individual cells within a massive and diverse population.
There are two types of detectors used in IFC:
- Multiplexed imaging devices: Charge Couple Devices (CCD) and Complementary Metal Oxide Semiconductor (CMOS)
- Single pixel photodetectors: Photomultiplier Tube (PMT) and Avalanche Photodiode (APD)
How CMOS camera-based Image Flow Cytometry (IFC) works
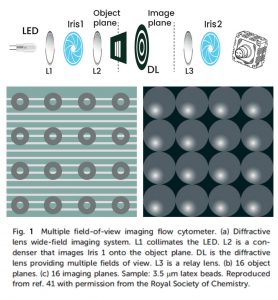
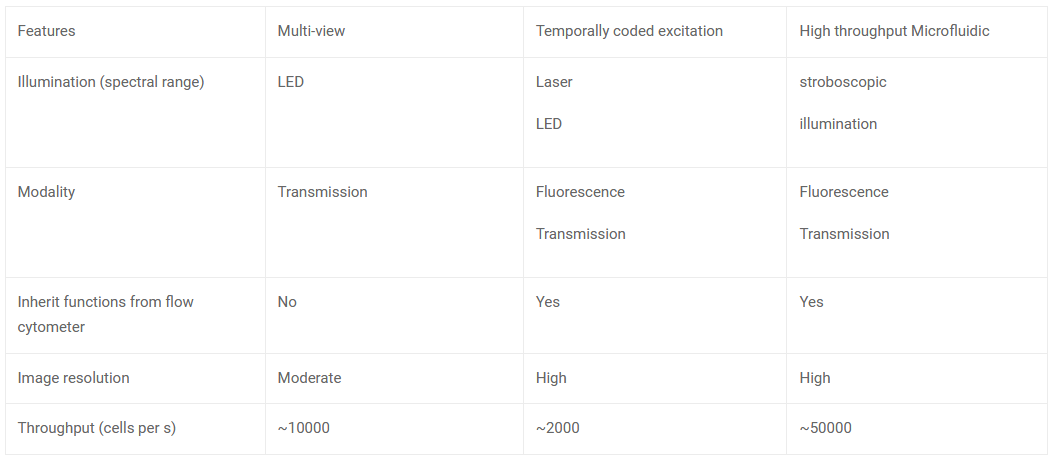
Multi-Field-Of-View Image Flow Cytometry (MIFC): MIFC gives a high throughput image by capturing multiple changes simultaneously. This is done by projecting multiple fields of view of parallel fluidic channels unto the CMOS sensor. However, MIFC works only in monochromatic mode.
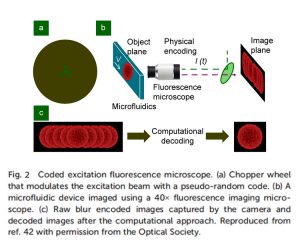
Temporally coded excitation: CMOS sensors can capture weak fluorescence signals by increasing the exposure time, but it may degrade image quality due to motion blur. The motion blur can be eliminated using temporally coded excitation. An alternative to continuous illumination is employing a chopper wheel to produce modulated excitation pulse sequences using a pseudo-random code. So, it allows the photos to be acquired and processed with a known code sequence and known point spread function before being de-blurred using computational procedures. A fluorescent image of a cell moving faster than the motion-blur velocity can be recreated using this method.
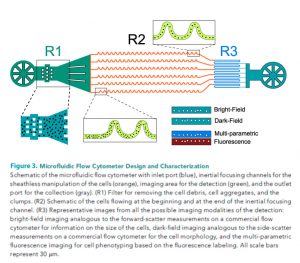
High throughput microfluidic flow cytometer: An ultra-high throughput imaging cytometer that uses stroboscopic lighting and inertial focusing allows sheathless manipulation of cells with no motion blur. The synchronisation of the camera frame rate and a parallel microfluidic design enables an analytical throughput of 85000 cells. The overall technique allows for the multi-parametric examination of cells, integrating both bright-field and dark-field imaging for size and morphology and multi-colour fluorescence detection for intracellular imaging.
Techniques used in CMOS camera-based Image Flow Cytometry (IFC)
e-con Systems offers a broad range of medical and life science camera solutions that can be seamlessly incorporated into applications like flow cytometers.