However, by partnering with experienced experts in power supply, these challenges and risks can be significantly mitigated. Indeed, this approach allows manufacturers to access high-quality, reliable and safe products, coupled to comprehensive and knowledgeable support geared to the medical standards of the day.
Standards deliver safety
People and power don’t always mix well, especially when the people are patients – either in medical facilities or, increasingly, at home. To ensure the safety of both patients and healthcare professionals, the sector is heavily regulated by a range of standards-based requirements and associated product testing.
Central to this regime is IEC 60601, comprising a suite of requirements specifically for electrical and electronic equipment used in healthcare.
One of the key safety concerns regarding medical devices is that the patient is often electrically connected to the device. One example of this is the conductive pads of an electrocardiograph. In IEC 60601, these are defined as ‘applied parts’ (AP) and are an important definition within the standard when defining a medical product’s overall requirements.
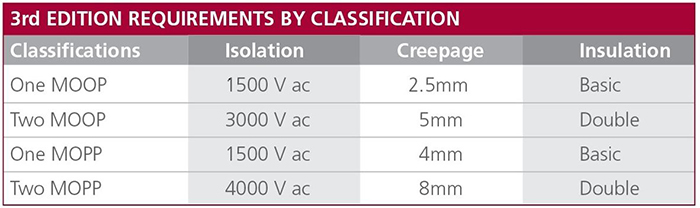
Above: Figure 1. Definitions of MOOPs and MOPPs for IEC 60601
Medical devices must incorporate at least one Means of Protection (MOP) to ensure both the patient, if connected via an AP, and operator are protected from the risk of electric shock, even under fault conditions. An MOP can be achieved through safety insulation, a protective earth, a defined creepage distance, an air gap, other protective impedances, or a combination of these techniques.
In the standard, operators and patients are treated differently, resulting in the classifications ‘Means of Operator Protection’ (MOOP) and ‘Means of Patient Protection’ (MOPP). One reason for this is that the patient may be physically connected via an AP and unconscious when the fault occurs. Because of this, the MOPP requirements are more stringent. Each term is defined in terms of an isolation voltage, creepage distance, and insulation level.
Evolving standards
IEC 60601 has evolved since first published almost 40 years ago. As power supplies and modules are not medical devices in their own right, the standards do not apply to them directly. However, medical device manufacturers would be unable to achieve standards compliance without power solutions designed with medical applications in mind.
Up until recently, it could safely be assumed that medical devices would be exclusively used in dedicated medical facilities. Such institutions provide dedicated clean power for use by their most sensitive medical devices. Nowadays, however, patients are demanding convenience – and resource-constrained medical facilities mean medical devices are increasingly used in the home. Here, issues such as electromagnetic compatibility (EMC) are of increased importance due to the prevalence of technologies such as Bluetooth and WiFi. Because of this, the latest version of the standard (4th edition) has changed the testing procedures and acceptance levels for EMC.
Another significant introduction has been the requirement to conduct risk assessment according to ISO 14971. Risk management is seen as a key component in demonstrating regulatory compliance for medical devices and ISO 14971 defines best practices for all stages of a medical device’s lifecycle.
The Medical Device Directive (MDD) adds further to the compliance burden, requiring manufacturers to implement a Quality Management System (QMS) compliant with ISO 13485. The primary requirement is for an organisation (such as a power supply manufacturer) to demonstrate its ability to meet both customer and regulatory requirements consistently.
Providing flexibility
The typical approach to achieving IEC 60601 compliance is to use an AC/DC power supply that is approved for medical use. However, BF rated applications also require the AP instrument to meet the 2 x MOPP rating. Many of the medical approved AC/DC power supplies on the market today do not have a 2 x MOPP rating and are not appropriate as a standalone power solution for applications where BF compliance is required. In these cases, an IEC 60601 approved, 2 x MOPP rated DC/DC converter will support BF compliance for the AP. Another common example is medical appliances which implement a battery back-up capability and must fulfil the 2 x MOPP rating during AC failure.
Medical appliances often require various DC voltages driving the AP instrument that are different to the main system DC voltage provided. In order to avoid sourcing a custom AC/DC power supply, this can be resolved by combining IEC 60601, 2 x MOPP rated DC/DC converters together with, for example, an ITE 60950 rated AC/DC power supply. Another option is to use 2 x MOPP, medical grade DC/DC converters for the AP instrument, even when the selected AC/DC power supply has IEC 60601 approval.
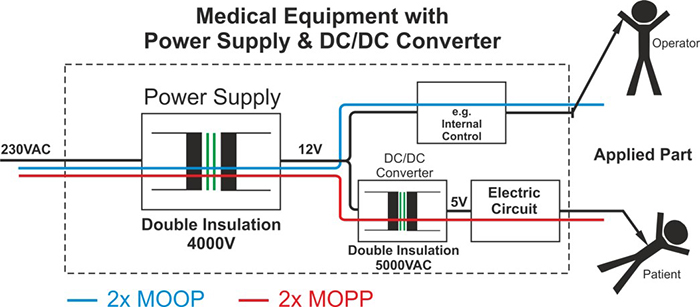
Above: Figure 2. Using a DC/DC converter to achieve 2 x MOPP protection
The primary requirements for 2 x MOPP (Figure 1) are 4,000V AC isolation, 8mm creepage distance and double insulation. Most commonly available DC/DC converters (including those that carry EN60950 approval) offer between 500V DC to around 1,600V DC of isolation and are, therefore, not suitable for medical applications. However, specialised DC/DC converters are available that will meet the requirements for AP when used in conjunction with such standard off-the-shelf power supplies.
By providing up to 5,000V AC of isolation, double insulation and 8mm of creepage distance through its galvanically-isolating transformer, a DC/DC converter can still protect the patient in the event of a failure of the main AC/DC power supply, thereby avoiding mains voltage levels appearing at any patient AP points.
Medically safe power solutions
At the heart of Traco’s approach to delivering world-class power solutions for the medical industry is the company’s transformer technology. Developed and honed over a long period, Traco’s approach ensures the required separation and isolation, while achieving sufficient coupling to allow the DC/DC converter to operate efficiently.
A low coupling capacitance between the primary and secondary transformer windings is an important aspect of the protection implementation. Values as low as 10-15pF ensure there is negligible transfer of current across the isolation barrier, providing patient protection in line with the requirements of the IEC 60601.
Traco also implements QMS in accordance with ISO 13485, which covers both its design and manufacturing processes. Additionally, further practices over and above the requirements of the standards ensure high levels of quality and safety within Traco products.

Above: Figure 3. Traco’s TPP 40 series is available as both open frame and enclosed
Industrial grade components are selected and sourced to ensure the ruggedness of the final product. As a result, devices intended for use in IT equipment are precluded by internal Traco policies. Workmanship is guaranteed through compliance with the international standard IPC-A-610 where Traco operates at level 3, the highest level of workmanship. Combined, these measures enable Traco to offer product warranties of up to five years on some products.
As a manufacturer of power solutions, rather than medical devices, Traco is not required to provide risk assessment data. However, Traco is compliant with ISO 14971 and provides risk assessment files covering crucial areas such as insulation breakdown, use while inverted, effects of fan failure, flammability, mechanical shock and more to their customers. The provision of this data contributes significantly to the risk assessment of its customers’ final medical products, saving both time and expense during their design process.
Product offering
Traco offers both AC/DC and DC/DC solutions for medical applications, all of which fulfil the 2 x MOPP requirement. Compliant with the EMC requirements of IEC 60601-1 (4th edition), they are suitable for all patient connected, applied parts medical devices (BF compliant). The AC/DC products range from small 5W PCB mounting modules, to a range of mid-power open frame designs, and enclosed power supplies offering power levels of up to 450W.
All of the PSUs offer universal mains input (85-264VAC / 120-370VDC) with active power factor correction (PFC) above 100W. The range comprises of single, dual and triple outputs, covering almost all application requirements.

Above: Figure 4. The 15W THM series is a PCB-mount medical DC/DC converter
Traco’s DC/DC converter range comprises PCB mounting modules with power levels from 2W to 30W. Devices offer both 2:1 and 4:1 input ranges with 5V, 12V, 24V and 48V nominal inputs available. Single and dual outputs are offered from 3.3VDC to ±15VDC.
All of Traco’s medically approved DC/DC converters offer 5,000VACrms of input-output isolation that is rated for a 250VACrms working voltage. This, along with leakage currents below 2μA, make them well suited for use in conjunction with non-approved AC/DC PSUs in safety critical medical applications.
Conclusion
International regulations governing products for use in the medical sector continue to become more and more stringent and navigating this minefield requires considerable technical knowledge and understanding. However, power supplies for these products are an area where manufacturers are increasingly partnering with other manufacturers who are focused solely on the provision of safe, compliant systems, allowing device manufacturers to dedicate themselves to innovation, quality and compliance in the technology areas in which they themselves specialise.