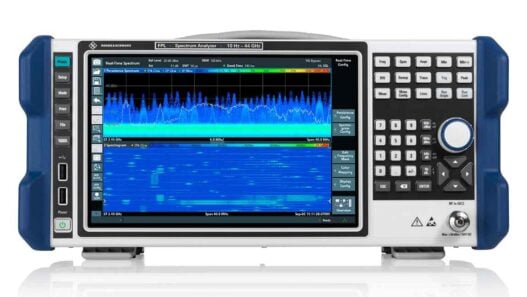
Additionally, it features capabilities such as InSpect integrated specimen imaging tool, helping to speed up and make biopsies less uncomfortable, and HD Breast Biopsy solution for one-click targeting of suspicious areas, allowing clinicians to do so with a 1 millimeter accuracy.
Breast compression is adjustable to match the needs and comfort thresholds of individual women, while achieving high quality imaging for diagnostic needs. For difficult to diagnose cases, the system can also be used for titanium contrast-enhanced mammography, potentially avoiding follow-up MRI scans.