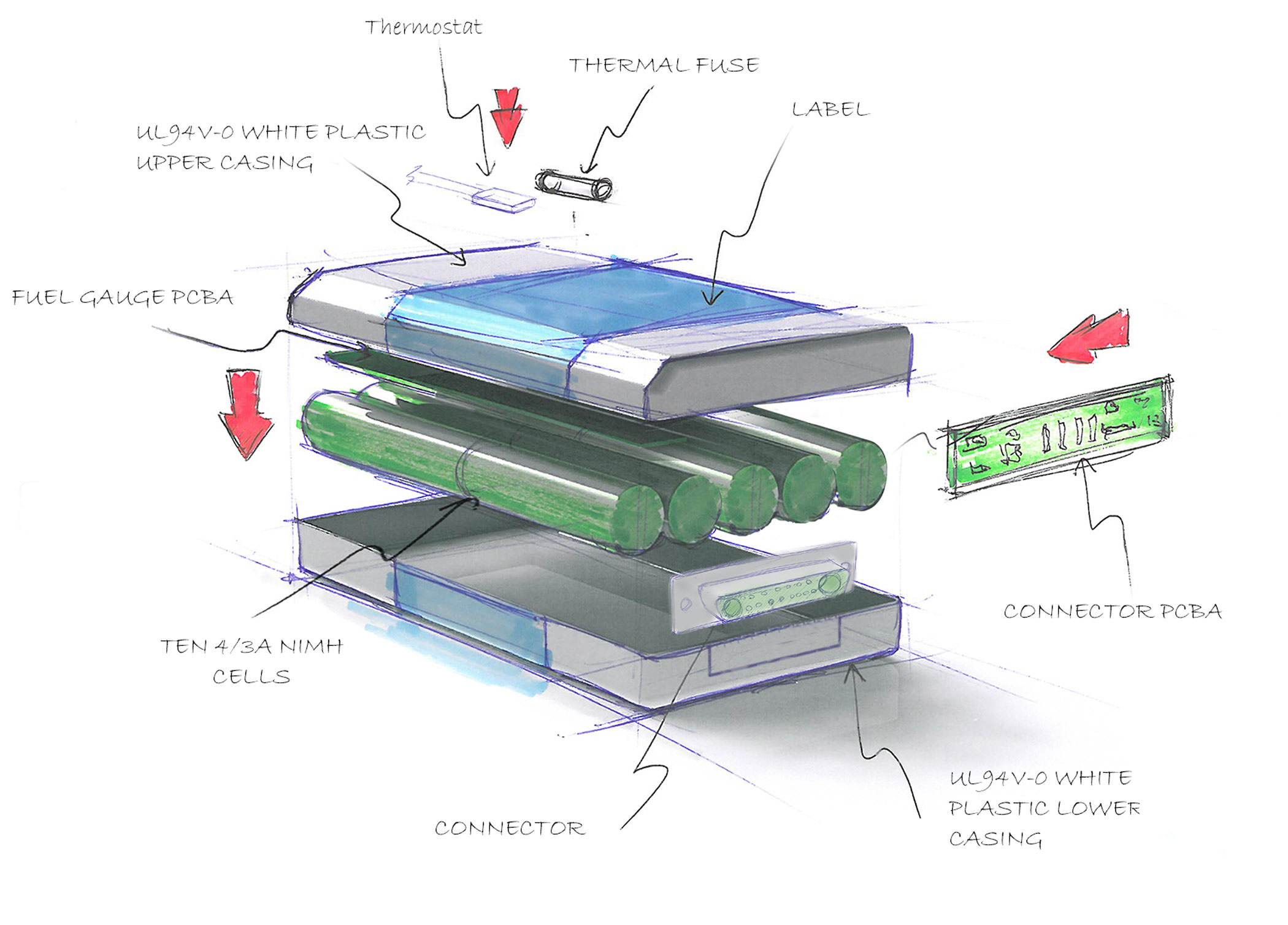
Designing medical devices for EMC
The MedTech world, along with the wider healthcare industry, has experienced a trend for more portable and wearable devices in the last few years. The problem is that these devices require lighter and equally portable batteries and chargers that use SMPS, a technology that is prone to higher EMI - not a useful characteristic for devices intended for use in life critical medical environments.
Anthony Robinson, Design Verification Engineer, Accutronics, looks at the implications of designing medical devices in light of changes to European IEC 60601-1-2 EMC regulations.
Battery technology has adapted rapidly to meet demand for smaller, lighter and more powerful devices. Keeping pace with this innovation, customers now demand increasingly lighter, more portable and faster chargers and Accutronics achieves this while providing a highly efficient and low loss power conversion by using SMPS. However, a by-product of the high frequency switching process is that EMI and RFI is increased.
As medical devices are used in professional and home healthcare environments it's important that EMI levels are minimised to eliminate the risk of chargers and power supplies causing interference and voltage fluctuation of life-critical devices, such as heart monitors, ECG and anaesthesia machines and ventilators. To regulate allowable limits of EMI, European legislation, IEC 60601-1 third edition, regulates the general requirements for the basic safety and essential performance of medical electrical equipment. IEC 60601-1-2 fourth edition is a collateral standard for electromagnetic disturbances and introduces specific requirements and tests.
As part of the design process at Accutronics, an initial customer requirement consultation allows us to develop a top-level medical battery specification. Customers are usually conscious of the fact that they want to create a highly stable device with a very low susceptibility to EMI. However, one of the biggest challenges we face is when battery integration is an afterthought, despite the fact that smart battery features and charging capability drastically affect the user experience and are equally as important as the ergonomic design process.
In order to overcome this challenge, design verification engineers must ensure that electronic design meshes perfectly with mechanical design as well as compliance and approval testing. Some of the more stringent aspects of the EMC regulations require very specific limits on insulation, electrical isolation, impedance, creepage as well as clearance tolerances.
Meeting these limits often requires optimising the layout of the PCB and designing the best use of critical tracks. It's also important to add additional filtering and snubbers to keep ringing and resonance at an absolute minimum. Although batteries are less noisy than chargers - the latter includes boost circuits - smart batteries contain microprocessors and data communication lines, adding a further source of RFI noise.
If a customer has already designed the external product, leaving a very limited footprint to design a battery and PCB, the battery can't be made any smaller without sacrificing energy density. This leaves just enough space to fit a blank PCB with no space for any components!
MedTech's adoption of wearable devices is driving demand for lighter batteries that meet EMC regulations
In situations like these, Accutronics uses its expertise and works alongside customers to produce a solution that involves adapting the design to optimise mechanical, electrical and ergonomic characteristics while minimising the transmission and susceptibility to EMI. Not an easy problem to rectify, but one that we’ve solved.
As we continue to experience the growth of portable and wearable devices, we'll see the cat and mouse game between batteries and chargers continue. Trends such as wireless inductive charging and rapid thirty second charging will continue to challenge design engineers to produce ever more novel methods of battery design.
To pre-empt these challenges, my advice to medical device OEMs is to prioritise battery selection. Placing it at the forefront of the design and development process will ensure that smart-feature innovation will be unhampered by electrical limitations, ultimately allowing OEMs to improve functionality while maintaining safety to EMC standards.