Flexible electronics in wearable cardiac monitoring technologies
In today's digital age, focus on digital health and the quantified self have led to the rapid rise of heart rate monitoring technologies through wearables such as fitness trackers and smartwatches. Such devices have already proven their ability in detecting hidden heart conditions such as tachycardia and atrial fibrillation in seemingly healthy people.
However, the majority of wrist-based devices currently serve only as an advance warning, they are not approved by the FDA for use as medical devices. Thus, cardiologists still need to use alternative technologies for their diagnostic and monitoring needs. Dr Nadia Tsao, Principal Analyst at IDTechEx, has recently discusses the topic of flexible electronics within the healthcare industry.
This is where flexible electronics comes in. Cardiac monitoring requires devices to make close contact with the skin, making devices that integrate flexible and even stretchable electronics ideal due to their ability to conform to the skin, the potential for a low profile, and overall patient comfort.
IDTechEx forecasts that flexible electronics in cardiac monitoring, deployed in electronic skin patches and electronic textiles, will be a $2bn market in the year 2030.
IDTechEx has been reporting on flexible electronics for the past decade and have recently published ‘Flexible Electronics in Healthcare 2020-2030’. In this article, IDTechEx describe how electronic skin patches and electronic textiles are used in cardiac monitoring.
Electronic skin patches
Electronic skin patches are wearable devices that contain electrical components which are attached to the skin, typically using an adhesive.
In cardiac monitoring, electronic skin patches present an interesting balance between the medical standard, which is a 12-lead ECG test, and consumer electronics such as smartwatches and fitness trackers. While electronic skin patches offer less data than can be obtained through a 12-lead ECG, they present more useful and accurate information than the optical technology used in smartwatches and fitness trackers, and offer continuous monitoring, unlike the 1-lead ECG in the newer smartwatch models.
Within medical applications, electronic skin patches bring increased mobility to the patient over the 12-lead test. The first step from the 12-lead ECG is the Holter monitor, a portable, wired, device designed to be used over 24-48 hours. However, this device remains unwieldy and intrusive.
To increase patient comfort, companies have developed cardiac monitoring patches in the form of one integrated device over a flexible substrate. By removing the wires and decreasing device footprint and weight, electronic skin patches are more comfortable to wear, and can be used for longer monitoring periods, up to 30 days. This longevity is critical in detecting events for patients who do not experience them daily. The next step for devices will be to incorporate printed electronics to manufacture integrated electrodes and devices with even close-fitting designs for greater patient comfort.
Overall, electronic skin patches for cardiac monitoring fill the gap between in-patient cardiac monitoring (accurate, safe, non-ambulatory, expensive), implantable cardiac monitors (accurate, less safe, ambulatory, expensive) and other wearable fitness devices (poor accuracy/no medical approval, safe, ambulatory, cheap).
However, the deployment of cardiac monitoring skin patches is not just limited to event monitoring or mobile cardiac telemetry. Outside of cardiac monitoring, electronic skin patches for monitoring of other diseases (e.g. respiratory), or general patient monitoring (in-patient, post-discharge, etc.) also contain cardiac monitoring capabilities.
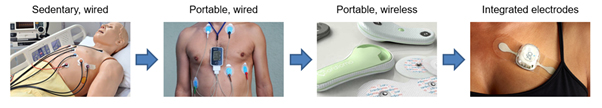
Above: Cardiac monitoring devices range from (left to right) the 12-lead ECG, Holter monitor, patch with snap fasteners, and patch with integrated electronics. // Source: IDTechEx research report, ‘Flexible Electronics in Healthcare 2020-2030’.
E-textiles
Electronic textiles, or e-textiles for short, are products that involve both electronic and textile components in a single device. The idea is to combine the functionality from electronic components with the comfort, aesthetics and ubiquity of textile products.
Smart clothing for sports used to be the major focus in the e-textiles industry - companies have made many attempts to develop mass-market products. Though e-textile companies may choose different strategies and technologies, the end products all have very similar functionalities such as tracking of activity, heart rate, respiratory rate, etc. There remains sporadic interest from apparel giants for sports applications, but many of the e-textile players have now shifted towards healthcare applications.
There is a close match between sports and medicine as the same vital signs are being detected and the same form factor (clothing) can be used. Within smart clothing, companies can design in 12- or even 15 leads for ECG readings, much more than the two to three offered by electronic skin patches. Moreover, smart clothing can be much more comfortable than electronic skin patches. The latter often causes discomfort through issues such as skin irritation. Despite the higher regulatory hurdle in healthcare vs sports, companies see the long-term benefit of e-textiles in healthcare. Smart clothing that is as comfortable as everyday clothing while still delivering medical-grade data will be key to automatic and continuous monitoring of patients going about their daily lives.
E-textiles are not just limited to clothing as a form factor, they may be incorporated into non-apparel textiles such as bed sheets, blankets, and even furniture. Regardless, the key for e-textile players today is validate their product through regulatory bodies such as the FDA, and to look into reimbursement for their products.
What’s next?
The COVID-19 pandemic has forced clinicians around the world to test out medical technologies to continue treating and monitoring their patients remotely. Though many physicians will eventually return to in-person practice, a fraction will continue utilising telemedicine and remote patient monitoring technologies.
Technologies such as electronic skin patches and e-textiles have much to offer to the healthcare system - remote patient monitoring has been shown to result in better outcomes, higher quality of care, and increased patient satisfaction. Healthcare systems will achieve cost savings through better management of patients and thus avoiding costly hospitalisation and emergency room visits. While reimbursement of remote patient monitoring technologies is moving in the right direction, it will remain a major hurdle for companies entering this space.


